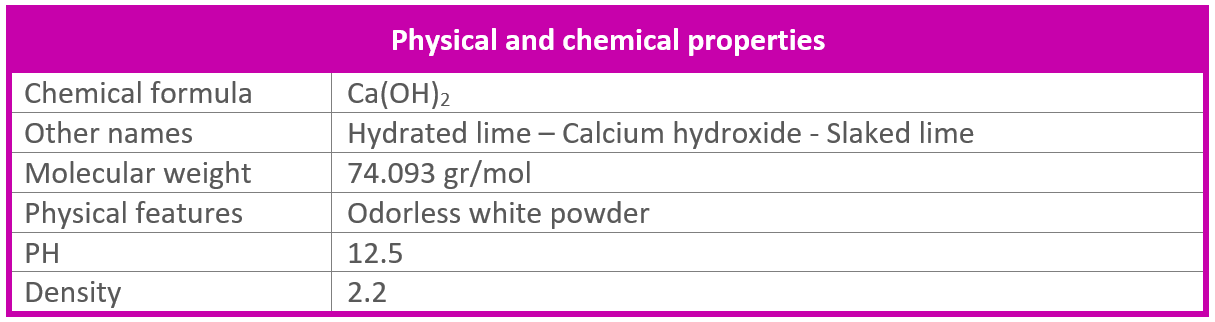
Calculate the weight of lime ( CaO) obtained by heating 2000 kg of 95% pure lime stone ( CaCO3) - Brainly.in

Calculate the weight of lime (CaO) obtained by heating 200 kg of 95% pure lime stone `(CaCO_(3)).` - YouTube

What mass of slaked lime would be required to decompose completely 4.28 g of ammonium chloride ?... - YouTube

The mass of lime (CaO) (in kg as the nearest integer) obtained by heating 200 kg of 95% pure limestone (CaCO3) is:

calculate the molar mass of quick lime (CaO) and baking powder (NaHCO3). (atomic masses; Na=23 u, H=1 u, - Brainly.in

The mass of lime (CaO) (in kg as the nearest integer) obtained by heating 200 kg of 95% pure limestone (CaCO3) is:

Write the formulae for the following and calculate the molecular mass for each one of them - YouTube